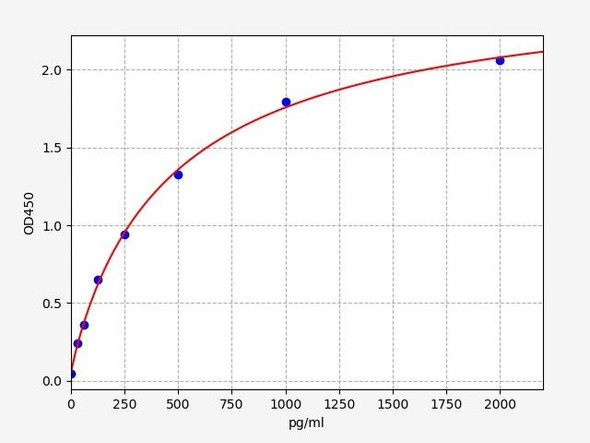
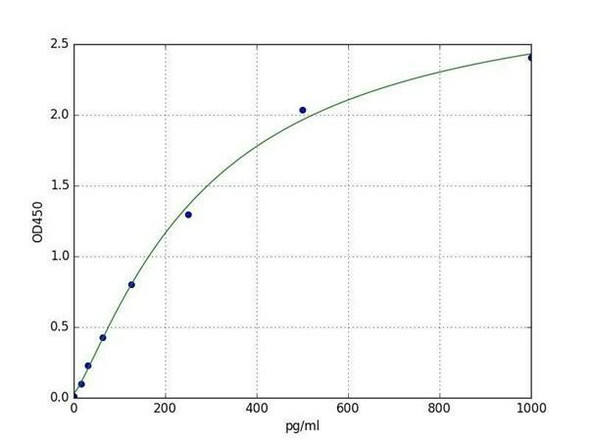
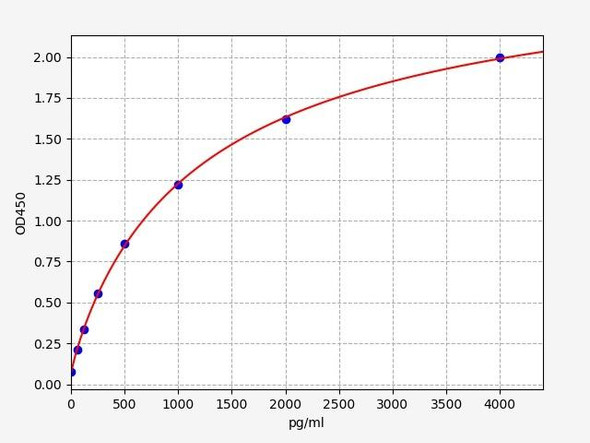
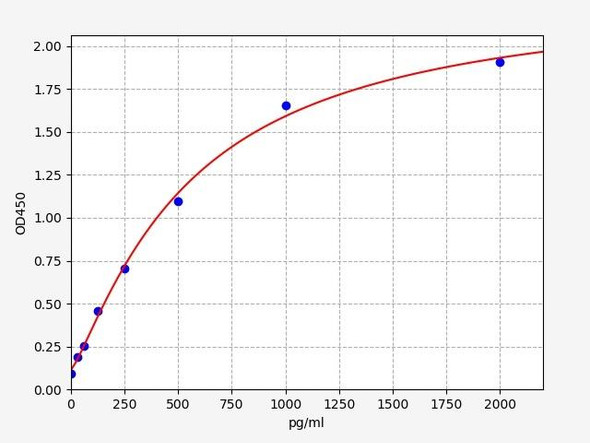
Rat IL-1B ELISA Kit (RTEB0339)
- SKU:
- RTEB0339
- Product Type:
- ELISA Kit
- Size:
- 96 Assays
- Uniprot:
- Q63264
- Range:
- 78-5000 pg/mL
- ELISA Type:
- Sandwich
- Synonyms:
- IL-1Beta, IL-1beta, IL1B, IL1-BETA, IL1F2, catabolin
- Reactivity:
- Rat
Description
| Product Name: | Rat IL-1B ELISA Kit |
| Product Code: | RTEB0339 |
| Alias: | Interleukin-1 beta, IL-1 beta, Il1b |
| Uniprot: | Q63264 |
| Reactivity: | Rat |
| Range: | 78-5000 pg/mL |
| Detection Method: | Sandwich |
| Size: | 96 Assay |
| Storage: | Please see kit components below for exact storage details |
| Note: | For research use only |
| UniProt Protein Function: | IL1B: Produced by activated macrophages, IL-1 stimulates thymocyte proliferation by inducing IL-2 release, B-cell maturation and proliferation, and fibroblast growth factor activity. IL-1 proteins are involved in the inflammatory response, being identified as endogenous pyrogens, and are reported to stimulate the release of prostaglandin and collagenase from synovial cells. Monomer. Belongs to the IL-1 family. |
| UniProt Protein Details: | Protein type:Cytokine Cellular Component: extracellular space; extracellular region; vesicle; secretory granule Molecular Function:protein domain specific binding; interleukin-1 receptor binding; cytokine activity Biological Process: positive regulation of granulocyte macrophage colony-stimulating factor production; negative regulation of MAP kinase activity; positive regulation of JNK activity; positive regulation of nitric oxide biosynthetic process; negative regulation of glutamate secretion; glycoprotein metabolic process; positive regulation of apoptosis; activation of MAPK activity; positive regulation of transcription, DNA-dependent; positive regulation of interleukin-2 biosynthetic process; response to glucocorticoid stimulus; germ cell programmed cell death; negative regulation of insulin receptor signaling pathway; positive regulation of glial cell differentiation; positive regulation of NF-kappaB import into nucleus; response to lipopolysaccharide; positive regulation of lipid catabolic process; fever; positive regulation of membrane protein ectodomain proteolysis; response to organic cyclic substance; response to carbohydrate stimulus; activation of NF-kappaB transcription factor; elevation of cytosolic calcium ion concentration; response to vitamin D; pentacyclic triterpenoid metabolic process; positive regulation of phagocytosis; positive regulation of T cell proliferation; positive regulation of astrocyte differentiation; response to drug; neutrophil chemotaxis; positive regulation of I-kappaB kinase/NF-kappaB cascade; positive regulation of heterotypic cell-cell adhesion; positive regulation of mitosis; positive regulation of interleukin-6 production; interleukin-1 beta production; social behavior; response to organic nitrogen; purine base metabolic process; positive regulation of angiogenesis; negative regulation of neuron differentiation; response to ethanol; response to heat; positive regulation of cell division; positive regulation of transcription factor activity; positive regulation of transcription from RNA polymerase II promoter; negative regulation of lipid metabolic process; leukocyte migration; response to peptide hormone stimulus; estrogen metabolic process; sequestering of triacylglycerol; wound healing; positive regulation of interleukin-6 biosynthetic process; response to morphine; positive regulation of JNK cascade; negative regulation of transcription from RNA polymerase II promoter; response to L-ascorbic acid; chronic inflammatory response to antigenic stimulus; positive regulation of stress-activated MAPK cascade; response to estradiol stimulus; negative regulation of neurogenesis; positive regulation of interleukin-8 production; negative regulation of cell proliferation; learning and/or memory; negative regulation of lipid catabolic process; hyaluronan biosynthetic process; protein kinase B signaling cascade; lipopolysaccharide-mediated signaling pathway; response to gamma radiation; regulation of I-kappaB kinase/NF-kappaB cascade; inflammatory response; response to nutrient; aging; cytokine and chemokine mediated signaling pathway; MAPKKK cascade; positive regulation of immature T cell proliferation in the thymus; response to ATP; memory; ovulation; polyketide metabolic process; positive regulation of interferon-gamma production; positive regulation of chemokine biosynthetic process; response to ozone; positive regulation of prostaglandin secretion; response to hypoxia; positive regulation of fever; immune response; positive regulation of protein amino acid phosphorylation; regulation of insulin secretion |
| NCBI Summary: | an inflammatory cytokine [RGD, Feb 2006] |
| UniProt Code: | Q63264 |
| NCBI GenInfo Identifier: | 2497335 |
| NCBI Gene ID: | 24494 |
| NCBI Accession: | Q63264.1 |
| UniProt Related Accession: | Q63264 |
| Molecular Weight: | 30,644 Da |
| NCBI Full Name: | Interleukin-1 beta |
| NCBI Synonym Full Names: | interleukin 1 beta |
| NCBI Official Symbol: | Il1b |
| NCBI Protein Information: | interleukin-1 beta; IL-1 beta |
| UniProt Protein Name: | Interleukin-1 beta |
| Protein Family: | Interleukin |
| UniProt Gene Name: | Il1b |
| UniProt Entry Name: | IL1B_RAT |
| Component | Quantity (96 Assays) | Storage |
| ELISA Microplate (Dismountable) | 8×12 strips | -20°C |
| Lyophilized Standard | 2 | -20°C |
| Sample Diluent | 20ml | -20°C |
| Assay Diluent A | 10mL | -20°C |
| Assay Diluent B | 10mL | -20°C |
| Detection Reagent A | 120µL | -20°C |
| Detection Reagent B | 120µL | -20°C |
| Wash Buffer | 30mL | 4°C |
| Substrate | 10mL | 4°C |
| Stop Solution | 10mL | 4°C |
| Plate Sealer | 5 | - |
Other materials and equipment required:
- Microplate reader with 450 nm wavelength filter
- Multichannel Pipette, Pipette, microcentrifuge tubes and disposable pipette tips
- Incubator
- Deionized or distilled water
- Absorbent paper
- Buffer resevoir
*Note: The below protocol is a sample protocol. Protocols are specific to each batch/lot. For the correct instructions please follow the protocol included in your kit.
Allow all reagents to reach room temperature (Please do not dissolve the reagents at 37°C directly). All the reagents should be mixed thoroughly by gently swirling before pipetting. Avoid foaming. Keep appropriate numbers of strips for 1 experiment and remove extra strips from microtiter plate. Removed strips should be resealed and stored at -20°C until the kits expiry date. Prepare all reagents, working standards and samples as directed in the previous sections. Please predict the concentration before assaying. If values for these are not within the range of the standard curve, users must determine the optimal sample dilutions for their experiments. We recommend running all samples in duplicate.
| Step | |
| 1. | Add Sample: Add 100µL of Standard, Blank, or Sample per well. The blank well is added with Sample diluent. Solutions are added to the bottom of micro ELISA plate well, avoid inside wall touching and foaming as possible. Mix it gently. Cover the plate with sealer we provided. Incubate for 120 minutes at 37°C. |
| 2. | Remove the liquid from each well, don't wash. Add 100µL of Detection Reagent A working solution to each well. Cover with the Plate sealer. Gently tap the plate to ensure thorough mixing. Incubate for 1 hour at 37°C. Note: if Detection Reagent A appears cloudy warm to room temperature until solution is uniform. |
| 3. | Aspirate each well and wash, repeating the process three times. Wash by filling each well with Wash Buffer (approximately 400µL) (a squirt bottle, multi-channel pipette,manifold dispenser or automated washer are needed). Complete removal of liquid at each step is essential. After the last wash, completely remove remaining Wash Buffer by aspirating or decanting. Invert the plate and pat it against thick clean absorbent paper. |
| 4. | Add 100µL of Detection Reagent B working solution to each well. Cover with the Plate sealer. Incubate for 60 minutes at 37°C. |
| 5. | Repeat the wash process for five times as conducted in step 3. |
| 6. | Add 90µL of Substrate Solution to each well. Cover with a new Plate sealer and incubate for 10-20 minutes at 37°C. Protect the plate from light. The reaction time can be shortened or extended according to the actual color change, but this should not exceed more than 30 minutes. When apparent gradient appears in standard wells, user should terminatethe reaction. |
| 7. | Add 50µL of Stop Solution to each well. If color change does not appear uniform, gently tap the plate to ensure thorough mixing. |
| 8. | Determine the optical density (OD value) of each well at once, using a micro-plate reader set to 450 nm. User should open the micro-plate reader in advance, preheat the instrument, and set the testing parameters. |
| 9. | After experiment, store all reagents according to the specified storage temperature respectively until their expiry. |
When carrying out an ELISA assay it is important to prepare your samples in order to achieve the best possible results. Below we have a list of procedures for the preparation of samples for different sample types.
| Sample Type | Protocol |
| Serum | If using serum separator tubes, allow samples to clot for 30 minutes at room temperature. Centrifuge for 10 minutes at 1,000x g. Collect the serum fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. If serum separator tubes are not being used, allow samples to clot overnight at 2-8°C. Centrifuge for 10 minutes at 1,000x g. Remove serum and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. |
| Plasma | Collect plasma using EDTA or heparin as an anticoagulant. Centrifuge samples at 4°C for 15 mins at 1000 × g within 30 mins of collection. Collect the plasma fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. Note: Over haemolysed samples are not suitable for use with this kit. |
| Urine & Cerebrospinal Fluid | Collect the urine (mid-stream) in a sterile container, centrifuge for 20 mins at 2000-3000 rpm. Remove supernatant and assay immediately. If any precipitation is detected, repeat the centrifugation step. A similar protocol can be used for cerebrospinal fluid. |
| Cell culture supernatant | Collect the cell culture media by pipette, followed by centrifugation at 4°C for 20 mins at 1500 rpm. Collect the clear supernatant and assay immediately. |
| Cell lysates | Solubilize cells in lysis buffer and allow to sit on ice for 30 minutes. Centrifuge tubes at 14,000 x g for 5 minutes to remove insoluble material. Aliquot the supernatant into a new tube and discard the remaining whole cell extract. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| Tissue homogenates | The preparation of tissue homogenates will vary depending upon tissue type. Rinse tissue with 1X PBS to remove excess blood & homogenize in 20ml of 1X PBS (including protease inhibitors) and store overnight at ≤ -20°C. Two freeze-thaw cycles are required to break the cell membranes. To further disrupt the cell membranes you can sonicate the samples. Centrifuge homogenates for 5 mins at 5000xg. Remove the supernatant and assay immediately or aliquot and store at -20°C or -80°C. |
| Tissue lysates | Rinse tissue with PBS, cut into 1-2 mm pieces, and homogenize with a tissue homogenizer in PBS. Add an equal volume of RIPA buffer containing protease inhibitors and lyse tissues at room temperature for 30 minutes with gentle agitation. Centrifuge to remove debris. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| Breast Milk | Collect milk samples and centrifuge at 10,000 x g for 60 min at 4°C. Aliquot the supernatant and assay. For long term use, store samples at -80°C. Minimize freeze/thaw cycles. |