Rat Cadherin-1 (Cdh1) ELISA Kit
- SKU:
- RTEB0012
- Product Type:
- ELISA Kit
- Size:
- 96 Assays
- Uniprot:
- Q9R0T4
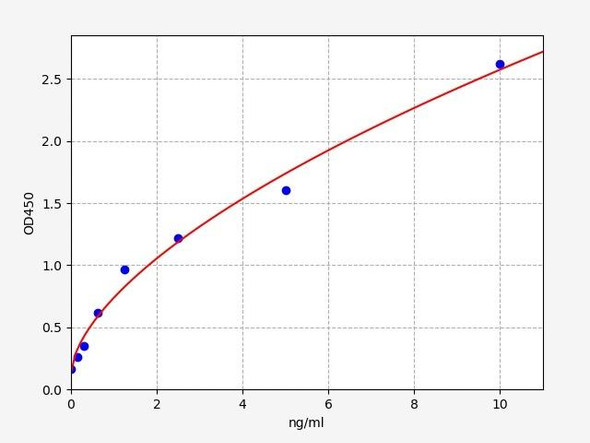
- Range:
- 0.156-10 ng/mL
- ELISA Type:
- Sandwich
- Synonyms:
- E-Cadherin, E-Cad, CDH1, Arc-1, CAD1, Cadherin-1, CD324, Cell-CAM120, ECAD, L-CAM, Uvomorulin, cadherin 1, E-cadherin, epithelial
- Reactivity:
- Rat
Description
| Product Name: | Rat Cadherin-1 (Cdh1) ELISA Kit |
| Product Code: | RTEB0012 |
| Alias: | Cadherin-1, Epithelial cadherin, E-cadherin, Uvomorulin, Cdh1, CD324 |
| Uniprot: | Q9R0T4 |
| Reactivity: | Rat |
| Range: | 0.156-10 ng/mL |
| Detection Method: | Sandwich |
| Size: | 96 Assay |
| Storage: | Please see kit components below for exact storage details |
| Note: | For research use only |
| UniProt Protein Function: | Function: Cadherins are calcium-dependent cell adhesion proteins. They preferentially interact with themselves in a homophilic manner in connecting cells; cadherins may thus contribute to the sorting of heterogeneous cell types. CDH1 is involved in mechanisms regulating cell-cell adhesions, mobility and proliferation of epithelial cells. Has a potent invasive suppressor role. It is a ligand for integrin alpha-E/beta-7. Ref.25E-Cad/CTF2 promotes non-amyloidogenic degradation of Abeta precursors. Has a strong inhibitory effect on APP C99 and C83 production. Ref.25 |
| UniProt Protein Details: | Subunit structure: Homodimer; disulfide-linked. Component of an E-cadherin/ catenin adhesion complex composed of at least E-cadherin/CDH1, beta-catenin/CTNNB1 or gamma-catenin/JUP, and potentially alpha-catenin/CTNNA1; the complex is located to adherens junctions. The stable association of CTNNA1 is controversial as CTNNA1 was shown not to bind to F-actin when assembled in the complex. Alternatively, the CTNNA1-containing complex may be linked to F-actin by other proteins such as LIMA1. Interaction with PSEN1, cleaves CDH1 resulting in the disassociation of cadherin-based adherens junctions (CAJs). Interacts with AJAP1, CTNND1 and DLGAP5 By similarity. Interacts with TBC1D2. Interacts with LIMA1. Interacts with CAV1. Interacts with the TRPV4 and CTNNB1 complex By similarity. Interacts with PIP5K1C. Interacts with RAB8B By similarity. Interacts with RAPGEF2 By similarity. Interacts with DDR1; this stabilizes CDH1 at the cell surface and inhibits its internalization. Ref.10 Ref.17 Ref.19 Ref.20 Ref.21 Ref.22 Ref.23 Ref.29 Ref.31 Ref.32 Subcellular location: Cell junction. Cell membrane; Single-pass type I membrane protein. Endosome. Golgi apparatus › trans-Golgi network. Note: Colocalizes with DLGAP5 at sites of cell-cell contact in intestinal epithelial cells. Anchored to actin microfilaments through association with alpha-, beta- and gamma-catenin. Sequential proteolysis induced by apoptosis or calcium influx, results in translocation from sites of cell-cell contact to the cytoplasm. Colocalizes with RAB11A endosomes during its transport from the Golgi apparatus to the plasma membrane. Ref.4 Ref.21 Ref.24 Ref.32 Tissue specificity: Non-neural epithelial tissues. Induction: Expression is repressed by MACROD1. Ref.26 Domain: Three calcium ions are usually bound at the interface of each cadherin domain and rigidify the connections, imparting a strong curvature to the full-length ectodomain. Ref.16 Post-translational modification: During apoptosis or with calcium influx, cleaved by a membrane-bound metalloproteinase (ADAM10), PS1/gamma-secretase and caspase-3 to produce fragments of about 38 kDa (E-CAD/CTF1), 33 kDa (E-CAD/CTF2) and 29 kDa (E-CAD/CTF3), respectively. Processing by the metalloproteinase, induced by calcium influx, causes disruption of cell-cell adhesion and the subsequent release of beta-catenin into the cytoplasm. The residual membrane-tethered cleavage product is rapidly degraded via an intracellular proteolytic pathway. Cleavage by caspase-3 releases the cytoplasmic tail resulting in disintegration of the actin microfilament system. The gamma-secretase-mediated cleavage promotes disassembly of adherens junctions. Ref.4 Ref.15 Ref.18N-glycosylation at Asn-637 is essential for expression, folding and trafficking.Ubiquitinated by a SCF complex containing SKP2, which requires prior phosphorylation by CK1/CSNK1A1. Ubiquitinated by CBLL1/HAKAI, requires prior phosphorylation at Tyr-754. Ref.34 Ref.35 Involvement in Disease: Hereditary diffuse gastric cancer (HDGC) [MIM:137215]: A cancer predisposition syndrome with increased susceptibility to diffuse gastric cancer. Diffuse gastric cancer is a malignant disease characterized by poorly differentiated infiltrating lesions resulting in thickening of the stomach. Malignant tumors start in the stomach, can spread to the esophagus or the small intestine, and can extend through the stomach wall to nearby lymph nodes and organs. It also can metastasize to other parts of the body.Note: The disease is caused by mutations affecting the gene represented in this entry. Heterozygous CDH1 germline mutations are responsible for familial cases of diffuse gastric cancer. Somatic mutations has also been found in patients with sporadic diffuse gastric cancer and lobular breast cancer. Ref.47 Ref.52Endometrial cancer (ENDMC) [MIM:608089]: A malignancy of endometrium, the mucous lining of the uterus. Most endometrial cancers are adenocarcinomas, cancers that begin in cells that make and release mucus and other fluids.Note: Disease susceptibility is associated with variations affecting the gene represented in this entry.Ovarian cancer (OC) [MIM:167000]: The term ovarian cancer defines malignancies originating from ovarian tissue. Although many histologic types of ovarian tumors have been described, epithelial ovarian carcinoma is the most common form. Ovarian cancers are often asymptomatic and the recognized signs and symptoms, even of late-stage disease, are vague. Consequently, most patients are diagnosed with advanced disease.Note: Disease susceptibility is associated with variations affecting the gene represented in this entry.Breast cancer, lobular (LBC) [MIM:137215]: A type of breast cancer that begins in the milk-producing glands (lobules) of the breast.Note: The gene represented in this entry may be involved in disease pathogenesis. Ref.27 Sequence similarities: Contains 5 cadherin domains. Sequence caution: The sequence AAA61259.1 differs from that shown. Reason: Frameshift at positions 16, 22, 25, 28, 31, 34, 52, 67, 73, 76, 94, 102, 633 and 636. |
| NCBI Summary: | This gene is a classical cadherin from the cadherin superfamily. The encoded protein is a calcium dependent cell-cell adhesion glycoprotein comprised of five extracellular cadherin repeats, a transmembrane region and a highly conserved cytoplasmic tail. Mutations in this gene are correlated with gastric, breast, colorectal, thyroid and ovarian cancer. Loss of function is thought to contribute to progression in cancer by increasing proliferation, invasion, and/or metastasis. The ectodomain of this protein mediates bacterial adhesion to mammalian cells and the cytoplasmic domain is required for internalization. Identified transcript variants arise from mutation at consensus splice sites. [provided by RefSeq, Jul 2008] |
| UniProt Code: | Q9R0T4 |
| NCBI GenInfo Identifier: | 148921649 |
| NCBI Gene ID: | 999 |
| NCBI Accession: | AAI46663.1 |
| UniProt Secondary Accession: | Q9R0T4,Q13799, Q14216, Q15855, Q16194, Q4PJ14, A8K1U7 |
| UniProt Related Accession: | P12830 |
| Molecular Weight: | |
| NCBI Full Name: | CDH1 protein |
| NCBI Synonym Full Names: | cadherin 1, type 1, E-cadherin (epithelial) |
| NCBI Official Symbol: | CDH1 |
| NCBI Official Synonym Symbols: | UVO; CDHE; ECAD; LCAM; Arc-1; CD324 |
| NCBI Protein Information: | cadherin-1; CAM 120/80; E-Cadherin; uvomorulin; cell-CAM 120/80; epithelial cadherin; cadherin 1, E-cadherin (epithelial); calcium-dependent adhesion protein, epithelial |
| UniProt Protein Name: | Cadherin-1 |
| UniProt Synonym Protein Names: | CAM 120/80; Epithelial cadherin; E-cadherin; Uvomorulin |
| Protein Family: | Cadherin |
| UniProt Gene Name: | CDH1 |
| UniProt Entry Name: | CADH1_HUMAN |
| Component | Quantity (96 Assays) | Storage |
| ELISA Microplate (Dismountable) | 8×12 strips | -20°C |
| Lyophilized Standard | 2 | -20°C |
| Sample Diluent | 20ml | -20°C |
| Assay Diluent A | 10mL | -20°C |
| Assay Diluent B | 10mL | -20°C |
| Detection Reagent A | 120µL | -20°C |
| Detection Reagent B | 120µL | -20°C |
| Wash Buffer | 30mL | 4°C |
| Substrate | 10mL | 4°C |
| Stop Solution | 10mL | 4°C |
| Plate Sealer | 5 | - |
Other materials and equipment required:
- Microplate reader with 450 nm wavelength filter
- Multichannel Pipette, Pipette, microcentrifuge tubes and disposable pipette tips
- Incubator
- Deionized or distilled water
- Absorbent paper
- Buffer resevoir
*Note: The below protocol is a sample protocol. Protocols are specific to each batch/lot. For the correct instructions please follow the protocol included in your kit.
Allow all reagents to reach room temperature (Please do not dissolve the reagents at 37°C directly). All the reagents should be mixed thoroughly by gently swirling before pipetting. Avoid foaming. Keep appropriate numbers of strips for 1 experiment and remove extra strips from microtiter plate. Removed strips should be resealed and stored at -20°C until the kits expiry date. Prepare all reagents, working standards and samples as directed in the previous sections. Please predict the concentration before assaying. If values for these are not within the range of the standard curve, users must determine the optimal sample dilutions for their experiments. We recommend running all samples in duplicate.
| Step | |
| 1. | Add Sample: Add 100µL of Standard, Blank, or Sample per well. The blank well is added with Sample diluent. Solutions are added to the bottom of micro ELISA plate well, avoid inside wall touching and foaming as possible. Mix it gently. Cover the plate with sealer we provided. Incubate for 120 minutes at 37°C. |
| 2. | Remove the liquid from each well, don't wash. Add 100µL of Detection Reagent A working solution to each well. Cover with the Plate sealer. Gently tap the plate to ensure thorough mixing. Incubate for 1 hour at 37°C. Note: if Detection Reagent A appears cloudy warm to room temperature until solution is uniform. |
| 3. | Aspirate each well and wash, repeating the process three times. Wash by filling each well with Wash Buffer (approximately 400µL) (a squirt bottle, multi-channel pipette,manifold dispenser or automated washer are needed). Complete removal of liquid at each step is essential. After the last wash, completely remove remaining Wash Buffer by aspirating or decanting. Invert the plate and pat it against thick clean absorbent paper. |
| 4. | Add 100µL of Detection Reagent B working solution to each well. Cover with the Plate sealer. Incubate for 60 minutes at 37°C. |
| 5. | Repeat the wash process for five times as conducted in step 3. |
| 6. | Add 90µL of Substrate Solution to each well. Cover with a new Plate sealer and incubate for 10-20 minutes at 37°C. Protect the plate from light. The reaction time can be shortened or extended according to the actual color change, but this should not exceed more than 30 minutes. When apparent gradient appears in standard wells, user should terminatethe reaction. |
| 7. | Add 50µL of Stop Solution to each well. If color change does not appear uniform, gently tap the plate to ensure thorough mixing. |
| 8. | Determine the optical density (OD value) of each well at once, using a micro-plate reader set to 450 nm. User should open the micro-plate reader in advance, preheat the instrument, and set the testing parameters. |
| 9. | After experiment, store all reagents according to the specified storage temperature respectively until their expiry. |
When carrying out an ELISA assay it is important to prepare your samples in order to achieve the best possible results. Below we have a list of procedures for the preparation of samples for different sample types.
| Sample Type | Protocol |
| Serum | If using serum separator tubes, allow samples to clot for 30 minutes at room temperature. Centrifuge for 10 minutes at 1,000x g. Collect the serum fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. If serum separator tubes are not being used, allow samples to clot overnight at 2-8°C. Centrifuge for 10 minutes at 1,000x g. Remove serum and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. |
| Plasma | Collect plasma using EDTA or heparin as an anticoagulant. Centrifuge samples at 4°C for 15 mins at 1000 × g within 30 mins of collection. Collect the plasma fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. Note: Over haemolysed samples are not suitable for use with this kit. |
| Urine & Cerebrospinal Fluid | Collect the urine (mid-stream) in a sterile container, centrifuge for 20 mins at 2000-3000 rpm. Remove supernatant and assay immediately. If any precipitation is detected, repeat the centrifugation step. A similar protocol can be used for cerebrospinal fluid. |
| Cell culture supernatant | Collect the cell culture media by pipette, followed by centrifugation at 4°C for 20 mins at 1500 rpm. Collect the clear supernatant and assay immediately. |
| Cell lysates | Solubilize cells in lysis buffer and allow to sit on ice for 30 minutes. Centrifuge tubes at 14,000 x g for 5 minutes to remove insoluble material. Aliquot the supernatant into a new tube and discard the remaining whole cell extract. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| Tissue homogenates | The preparation of tissue homogenates will vary depending upon tissue type. Rinse tissue with 1X PBS to remove excess blood & homogenize in 20ml of 1X PBS (including protease inhibitors) and store overnight at ≤ -20°C. Two freeze-thaw cycles are required to break the cell membranes. To further disrupt the cell membranes you can sonicate the samples. Centrifuge homogenates for 5 mins at 5000xg. Remove the supernatant and assay immediately or aliquot and store at -20°C or -80°C. |
| Tissue lysates | Rinse tissue with PBS, cut into 1-2 mm pieces, and homogenize with a tissue homogenizer in PBS. Add an equal volume of RIPA buffer containing protease inhibitors and lyse tissues at room temperature for 30 minutes with gentle agitation. Centrifuge to remove debris. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| Breast Milk | Collect milk samples and centrifuge at 10,000 x g for 60 min at 4°C. Aliquot the supernatant and assay. For long term use, store samples at -80°C. Minimize freeze/thaw cycles. |