Mouse Apoptosis regulator Bcl-2 (Bcl2) ELISA Kit
- SKU:
- MOEB0590
- Product Type:
- ELISA Kit
- Size:
- 96 Assays
- Uniprot:
- P10417
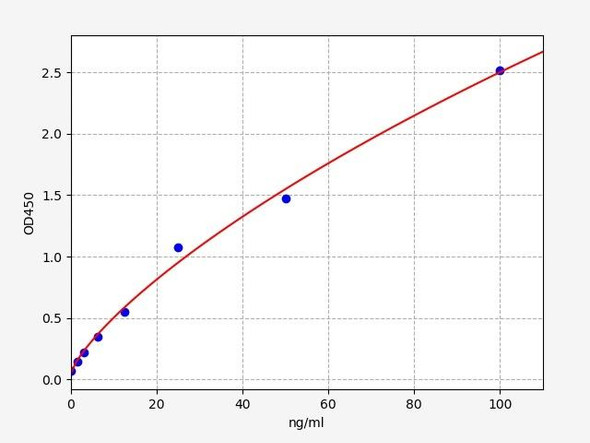
- Range:
- 0.156-10 ng/mL
- ELISA Type:
- Sandwich
- Synonyms:
- Bcl2, Bcl-2, apoptosis regulator Bcl-2, B-cell CLL, lymphoma 2
- Reactivity:
- Mouse
Description
| Product Name: | Mouse Apoptosis regulator Bcl-2 (Bcl2) ELISA Kit |
| Product Code: | MOEB0590 |
| Alias: | Apoptosis regulator Bcl-2, Bcl2, Bcl-2 |
| Uniprot: | P10417 |
| Reactivity: | Mouse |
| Range: | 0.156-10 ng/mL |
| Detection Method: | Sandwich |
| Size: | 96 Assay |
| Storage: | Please see kit components below for exact storage details |
| Note: | For research use only |
| UniProt Protein Function: | Bcl-2: a antiapoptotic member of the Bcl-2 family. Regulates cell death by controlling the mitochondrial membrane permeability. Inhibits caspase activity either by preventing the release of cytochrome c from the mitochondria and/or by binding to the apoptosis-activating factor (APAF-1). Phosphorylation by JNKs may increase its antiapoptotic functions. |
| UniProt Protein Details: | Protein type:Oncoprotein; Autophagy; Apoptosis; Membrane protein, integral Cellular Component: cytoplasm; cytosol; endoplasmic reticulum; endoplasmic reticulum membrane; integral to membrane; intracellular; membrane; mitochondrial membrane; mitochondrial outer membrane; mitochondrion; myelin sheath; nuclear membrane; nucleus; perinuclear region of cytoplasm; pore complex Molecular Function:BH domain binding; BH3 domain binding; channel activity; channel inhibitor activity; identical protein binding; protease binding; protein binding; protein heterodimerization activity; protein homodimerization activity; protein phosphatase 2A binding; protein phosphatase binding; transcription factor binding; ubiquitin protein ligase binding Biological Process: actin filament organization; apoptosis; apoptotic mitochondrial changes; axon regeneration; axonogenesis; B cell differentiation; B cell homeostasis; B cell lineage commitment; B cell proliferation; B cell receptor signaling pathway; behavioral fear response; CD8-positive, alpha-beta T cell lineage commitment; cell aging; cell growth; cell morphogenesis; cell proliferation; cell-cell adhesion; cellular calcium ion homeostasis; cellular response to glucose starvation; cochlear nucleus development; defense response to virus; developmental growth; digestive tract morphogenesis; DNA damage response, signal transduction resulting in induction of apoptosis; ear development; endoplasmic reticulum calcium ion homeostasis; focal adhesion formation; gland morphogenesis; glomerulus development; growth; hair follicle morphogenesis; hemopoiesis; homeostasis of number of cells within a tissue; immune system development; induction of apoptosis by oxidative stress; induction of apoptosis via death domain receptors; kidney development; leukocyte homeostasis; lymphocyte homeostasis; lymphoid progenitor cell differentiation; male gonad development; melanin metabolic process; melanocyte differentiation; mesenchymal cell development; metanephros development; negative regulation of apoptosis; negative regulation of autophagy; negative regulation of cell growth; negative regulation of cell migration; negative regulation of cell proliferation; negative regulation of cellular pH reduction; negative regulation of mitotic cell cycle; negative regulation of myeloid cell apoptosis; negative regulation of neuron apoptosis; negative regulation of ossification; negative regulation of osteoblast proliferation; negative regulation of retinal cell programmed cell death; neuron apoptosis; oocyte development; organ growth; organ morphogenesis; ossification; ovarian follicle development; peptidyl-serine phosphorylation; peptidyl-threonine phosphorylation; pigment granule organization and biogenesis; pigmentation; pigmentation during development; positive regulation of B cell proliferation; positive regulation of catalytic activity; positive regulation of cell growth; positive regulation of cell proliferation; positive regulation of melanocyte differentiation; positive regulation of multicellular organism growth; positive regulation of neuron maturation; positive regulation of peptidyl-serine phosphorylation; positive regulation of pigmentation; positive regulation of skeletal muscle fiber development; positive regulation of smooth muscle cell migration; post-embryonic development; protein amino acid dephosphorylation; protein polyubiquitination; regulation of apoptosis; regulation of autophagy; regulation of calcium ion transport; regulation of catalytic activity; regulation of cell cycle; regulation of cell-matrix adhesion; regulation of gene expression; regulation of mitochondrial membrane permeability; regulation of mitochondrial membrane potential; regulation of nitrogen utilization; regulation of pigmentation during development; regulation of programmed cell death; regulation of protein heterodimerization activity; regulation of protein homodimerization activity; regulation of protein localization; regulation of protein stability; regulation of transmembrane transporter activity; regulation of viral genome replication; release of cytochrome c from mitochondria; renal system process; response to acid; response to cytokine stimulus; response to DNA damage stimulus; response to drug; response to gamma radiation; response to glucocorticoid stimulus; response to hydrogen peroxide; response to iron ion; response to nicotine; response to oxidative stress; response to steroid hormone stimulus; response to toxin; response to UV-B; spleen development; T cell differentiation; T cell differentiation in the thymus; T cell homeostasis; T cell lineage commitment; thymus development; transmembrane transport; ureteric bud branching; ureteric bud development |
| NCBI Summary: | This gene encodes a member of the B cell lymphoma 2 protein family. Members of this family regulate cell death in multiple cell types and can have either proapoptotic or antiapoptotic activities. The protein encoded by this gene inhibits mitochondrial-mediated apoptosis. This protein is an integral outer mitochondrial membrane protein that functions as part of signaling pathway that controls mitochondrial permeability in response to apoptotic stimuli. This protein may also play a role in neuron cell survival and autophagy. Abnormal expression and chromosomal translocations of this gene are associated with cancer progression in numerous tissues. Alternate splicing results in multiple transcript variants. [provided by RefSeq, Sep 2015] |
| UniProt Code: | P10417 |
| NCBI GenInfo Identifier: | 408360316 |
| NCBI Gene ID: | 12043 |
| NCBI Accession: | P10417.3 |
| UniProt Secondary Accession: | P10417,P10418, Q4VBF6, |
| UniProt Related Accession: | P10417 |
| Molecular Weight: | 22,281 Da |
| NCBI Full Name: | Apoptosis regulator Bcl-2 |
| NCBI Synonym Full Names: | B cell leukemia/lymphoma 2 |
| NCBI Official Symbol: | Bcl2Â Â |
| NCBI Official Synonym Symbols: | Bcl-2; AW986256; C430015F12Rik; D630044D05Rik; D830018M01Rik  |
| NCBI Protein Information: | apoptosis regulator Bcl-2 |
| UniProt Protein Name: | Apoptosis regulator Bcl-2 |
| Protein Family: | Bcl2-associated agonist of cell death |
| UniProt Gene Name: | Bcl2Â Â |
| UniProt Entry Name: | BCL2_MOUSE |
| Component | Quantity (96 Assays) | Storage |
| ELISA Microplate (Dismountable) | 8×12 strips | -20°C |
| Lyophilized Standard | 2 | -20°C |
| Sample Diluent | 20ml | -20°C |
| Assay Diluent A | 10mL | -20°C |
| Assay Diluent B | 10mL | -20°C |
| Detection Reagent A | 120µL | -20°C |
| Detection Reagent B | 120µL | -20°C |
| Wash Buffer | 30mL | 4°C |
| Substrate | 10mL | 4°C |
| Stop Solution | 10mL | 4°C |
| Plate Sealer | 5 | - |
Other materials and equipment required:
- Microplate reader with 450 nm wavelength filter
- Multichannel Pipette, Pipette, microcentrifuge tubes and disposable pipette tips
- Incubator
- Deionized or distilled water
- Absorbent paper
- Buffer resevoir
*Note: The below protocol is a sample protocol. Protocols are specific to each batch/lot. For the correct instructions please follow the protocol included in your kit.
Allow all reagents to reach room temperature (Please do not dissolve the reagents at 37°C directly). All the reagents should be mixed thoroughly by gently swirling before pipetting. Avoid foaming. Keep appropriate numbers of strips for 1 experiment and remove extra strips from microtiter plate. Removed strips should be resealed and stored at -20°C until the kits expiry date. Prepare all reagents, working standards and samples as directed in the previous sections. Please predict the concentration before assaying. If values for these are not within the range of the standard curve, users must determine the optimal sample dilutions for their experiments. We recommend running all samples in duplicate.
| Step | |
| 1. | Add Sample: Add 100µL of Standard, Blank, or Sample per well. The blank well is added with Sample diluent. Solutions are added to the bottom of micro ELISA plate well, avoid inside wall touching and foaming as possible. Mix it gently. Cover the plate with sealer we provided. Incubate for 120 minutes at 37°C. |
| 2. | Remove the liquid from each well, don't wash. Add 100µL of Detection Reagent A working solution to each well. Cover with the Plate sealer. Gently tap the plate to ensure thorough mixing. Incubate for 1 hour at 37°C. Note: if Detection Reagent A appears cloudy warm to room temperature until solution is uniform. |
| 3. | Aspirate each well and wash, repeating the process three times. Wash by filling each well with Wash Buffer (approximately 400µL) (a squirt bottle, multi-channel pipette,manifold dispenser or automated washer are needed). Complete removal of liquid at each step is essential. After the last wash, completely remove remaining Wash Buffer by aspirating or decanting. Invert the plate and pat it against thick clean absorbent paper. |
| 4. | Add 100µL of Detection Reagent B working solution to each well. Cover with the Plate sealer. Incubate for 60 minutes at 37°C. |
| 5. | Repeat the wash process for five times as conducted in step 3. |
| 6. | Add 90µL of Substrate Solution to each well. Cover with a new Plate sealer and incubate for 10-20 minutes at 37°C. Protect the plate from light. The reaction time can be shortened or extended according to the actual color change, but this should not exceed more than 30 minutes. When apparent gradient appears in standard wells, user should terminatethe reaction. |
| 7. | Add 50µL of Stop Solution to each well. If color change does not appear uniform, gently tap the plate to ensure thorough mixing. |
| 8. | Determine the optical density (OD value) of each well at once, using a micro-plate reader set to 450 nm. User should open the micro-plate reader in advance, preheat the instrument, and set the testing parameters. |
| 9. | After experiment, store all reagents according to the specified storage temperature respectively until their expiry. |
When carrying out an ELISA assay it is important to prepare your samples in order to achieve the best possible results. Below we have a list of procedures for the preparation of samples for different sample types.
| Sample Type | Protocol |
| Serum | If using serum separator tubes, allow samples to clot for 30 minutes at room temperature. Centrifuge for 10 minutes at 1,000x g. Collect the serum fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. If serum separator tubes are not being used, allow samples to clot overnight at 2-8°C. Centrifuge for 10 minutes at 1,000x g. Remove serum and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. |
| Plasma | Collect plasma using EDTA or heparin as an anticoagulant. Centrifuge samples at 4°C for 15 mins at 1000 × g within 30 mins of collection. Collect the plasma fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. Note: Over haemolysed samples are not suitable for use with this kit. |
| Urine & Cerebrospinal Fluid | Collect the urine (mid-stream) in a sterile container, centrifuge for 20 mins at 2000-3000 rpm. Remove supernatant and assay immediately. If any precipitation is detected, repeat the centrifugation step. A similar protocol can be used for cerebrospinal fluid. |
| Cell culture supernatant | Collect the cell culture media by pipette, followed by centrifugation at 4°C for 20 mins at 1500 rpm. Collect the clear supernatant and assay immediately. |
| Cell lysates | Solubilize cells in lysis buffer and allow to sit on ice for 30 minutes. Centrifuge tubes at 14,000 x g for 5 minutes to remove insoluble material. Aliquot the supernatant into a new tube and discard the remaining whole cell extract. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| Tissue homogenates | The preparation of tissue homogenates will vary depending upon tissue type. Rinse tissue with 1X PBS to remove excess blood & homogenize in 20ml of 1X PBS (including protease inhibitors) and store overnight at ≤ -20°C. Two freeze-thaw cycles are required to break the cell membranes. To further disrupt the cell membranes you can sonicate the samples. Centrifuge homogenates for 5 mins at 5000xg. Remove the supernatant and assay immediately or aliquot and store at -20°C or -80°C. |
| Tissue lysates | Rinse tissue with PBS, cut into 1-2 mm pieces, and homogenize with a tissue homogenizer in PBS. Add an equal volume of RIPA buffer containing protease inhibitors and lyse tissues at room temperature for 30 minutes with gentle agitation. Centrifuge to remove debris. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| Breast Milk | Collect milk samples and centrifuge at 10,000 x g for 60 min at 4°C. Aliquot the supernatant and assay. For long term use, store samples at -80°C. Minimize freeze/thaw cycles. |