Human SIRT2 Recombinant Protein (RPPB4692)
- SKU:
- RPPB4692
- Product type:
- Recombinant Protein
- Size:
- 5ug
- Species:
- Human
- Target:
- SIRT2
- Synonyms:
- Sirtuin 2
- SIR2L2
- SIR2-like protein 2
- NAD-dependent deacetylase sirtuin-2
- Source:
- Escherichia Coli
- Uniprot:
- Q8IXJ6
Frequently bought together:
Description
| Product Name: | Human SIRT2 Recombinant Protein |
| Product Code: | RPPB4692 |
| Size: | 5µg |
| Species: | Human |
| Target: | SIRT2 |
| Synonyms: | Sirtuin 2, SIR2L2, SIR2-like protein 2, NAD-dependent deacetylase sirtuin-2, Silent Information Regulator 2. |
| Source: | Escherichia Coli |
| Physical Appearance: | Sterile Filtered clear solution. |
| Formulation: | The SIRT2 protein solution (0.25mg/1ml) is formulated in 20mM Tris-HCl buffer (pH8.0) 2mM DTT, 200mM NaCl, 0.5mM EDTA and 30% glycerol. |
| Stability: | Store at 4°C if entire vial will be used within 2-4 weeks. Store, frozen at -20°C for longer periods of time.Please avoid freeze thaw cycles. |
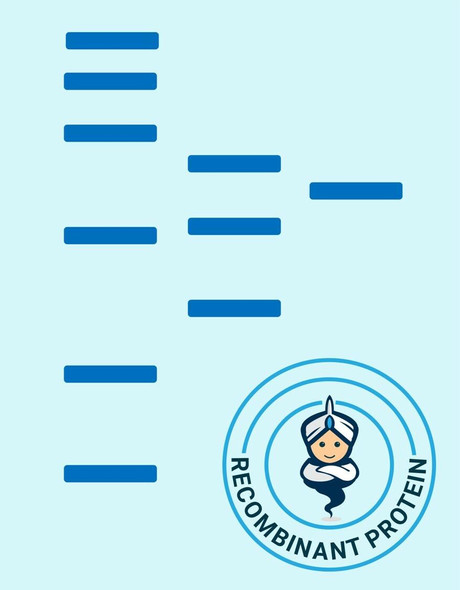
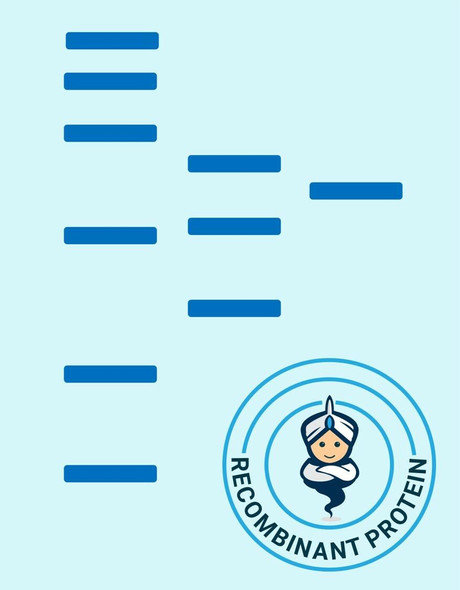
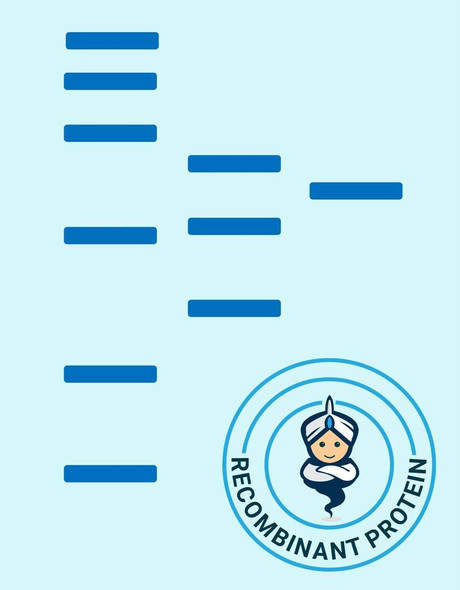
| Purity: | Greater than 90% as determined by SDS-PAGE. |
| Amino Acid Sequence: | MGSSHHHHHH SSGLVPRGSH MDFLRNLFSQ TLSLGSQKER LLDELTLEGV ARYMQSERCR RVICLVGAGI STSAGIPDFR SPSTGLYDNL EKYHLPYPEA IFEISYFKKH PEPFFALAKE LYPGQFKPTI CHYFMRLLKD KGLLLRCYTQ NIDTLERIAG LEQEDLVEAH GTFYTSHCVS ASCRHEYPLS WMKEKIFSEV TPKCEDCQSL VKPDIVFFGE SLPARFFSCM QSDFLKVDLL LVMGTSLQVQ PFASLISKAP LSTPRLLINK EKAGQSDPFL GMIMGLGGGM DFDSKKAYRD VAWLGECDQG CLALAELLGW KKELEDLVRR EHASIDAQSG AGVPNPSTSA SPKKSPPPAK DEARTTEREK PQ |
SIRT2 belongs to the sirtuin family of proteins, homologs to the yeast Sir2 protein. Proteins of the sirtuin family are characterized by a sirtuin core domain and grouped into four classes and take part in various processes, including transcriptional regulation, cell cycle progression, DNA-damage repair and aging. SIRT2 is a NAD-dependent deacetylase, which deacetylates the 'Lys-40' of alpha-tubulin.
SIRT2 produced in E.Coli is a single, non-glycosylated polypeptide chain containing 372 amino acids (1-352a.a.) and having a molecular mass of 41.7kDa.SIRT2 is fused to a 20 amino acid His-tag at N-terminus & purified by proprietary chromatographic techniques.
| UniProt Protein Function: | NAD-dependent protein deacetylase, which deacetylates internal lysines on histone and alpha-tubulin as well as many other proteins such as key transcription factors. Participates in the modulation of multiple and diverse biological processes such as cell cycle control, genomic integrity, microtubule dynamics, cell differentiation, metabolic networks, and autophagy. Plays a major role in the control of cell cycle progression and genomic stability. Functions in the antephase checkpoint preventing precocious mitotic entry in response to microtubule stress agents, and hence allowing proper inheritance of chromosomes. Positively regulates the anaphase promoting complex/cyclosome (APC/C) ubiquitin ligase complex activity by deacetylating CDC20 and FZR1, then allowing progression through mitosis. Associates both with chromatin at transcriptional start sites (TSSs) and enhancers of active genes. Plays a role in cell cycle and chromatin compaction through epigenetic modulation of the regulation of histone H4 'Lys-20' methylation (H4K20me1) during early mitosis. Specifically deacetylates histone H4 at 'Lys-16' (H4K16ac) between the G2/M transition and metaphase enabling H4K20me1 deposition by KMT5A leading to ulterior levels of H4K20me2 and H4K20me3 deposition throughout cell cycle, and mitotic S-phase progression. Deacetylates KMT5A modulating KMT5A chromatin localization during the mitotic stress response. Deacetylates also histone H3 at 'Lys-57' (H3K56ac) during the mitotic G2/M transition. Upon bacterium Listeria monocytogenes infection, deacetylates 'Lys-18' of histone H3 in a receptor tyrosine kinase MET- and PI3K/Akt-dependent manner, thereby inhibiting transcriptional activity and promoting late stages of listeria infection. During oocyte meiosis progression, may deacetylate histone H4 at 'Lys-16' (H4K16ac) and alpha-tubulin, regulating spindle assembly and chromosome alignment by influencing microtubule dynamics and kinetochore function. Deacetylates alpha-tubulin at 'Lys-40' and hence controls neuronal motility, oligodendroglial cell arbor projection processes and proliferation of non-neuronal cells. Phosphorylation at Ser-368 by a G1/S-specific cyclin E-CDK2 complex inactivates SIRT2-mediated alpha-tubulin deacetylation, negatively regulating cell adhesion, cell migration and neurite outgrowth during neuronal differentiation. Deacetylates PARD3 and participates in the regulation of Schwann cell peripheral myelination formation during early postnatal development and during postinjury remyelination. Involved in several cellular metabolic pathways. Plays a role in the regulation of blood glucose homeostasis by deacetylating and stabilizing phosphoenolpyruvate carboxykinase PCK1 activity in response to low nutrient availability. Acts as a key regulator in the pentose phosphate pathway (PPP) by deacetylating and activating the glucose-6-phosphate G6PD enzyme, and therefore, stimulates the production of cytosolic NADPH to counteract oxidative damage. Maintains energy homeostasis in response to nutrient deprivation as well as energy expenditure by inhibiting adipogenesis and promoting lipolysis. Attenuates adipocyte differentiation by deacetylating and promoting FOXO1 interaction to PPARG and subsequent repression of PPARG-dependent transcriptional activity. Plays a role in the regulation of lysosome-mediated degradation of protein aggregates by autophagy in neuronal cells. Deacetylates FOXO1 in response to oxidative stress or serum deprivation, thereby negatively regulating FOXO1-mediated autophagy. Deacetylates a broad range of transcription factors and co-regulators regulating target gene expression. Deacetylates transcriptional factor FOXO3 stimulating the ubiquitin ligase SCF(SKP2)-mediated FOXO3 ubiquitination and degradation. Deacetylates HIF1A and therefore promotes HIF1A degradation and inhibition of HIF1A transcriptional activity in tumor cells in response to hypoxia. Deacetylates RELA in the cytoplasm inhibiting NF-kappaB-dependent transcription activation upon TNF-alpha stimulation. Inhibits transcriptional activation by deacetylating p53/TP53 and EP300. Deacetylates also EIF5A. Functions as a negative regulator on oxidative stress-tolerance in response to anoxia-reoxygenation conditions. Plays a role as tumor suppressor. |
| NCBI Summary: | This gene encodes a member of the sirtuin family of proteins, homologs to the yeast Sir2 protein. Members of the sirtuin family are characterized by a sirtuin core domain and grouped into four classes. The functions of human sirtuins have not yet been determined; however, yeast sirtuin proteins are known to regulate epigenetic gene silencing and suppress recombination of rDNA. Studies suggest that the human sirtuins may function as intracellular regulatory proteins with mono-ADP-ribosyltransferase activity. The protein encoded by this gene is included in class I of the sirtuin family. Several transcript variants are resulted from alternative splicing of this gene. [provided by RefSeq, Jul 2010] |
| UniProt Code: | Q8IXJ6 |
| NCBI GenInfo Identifier: | 38258608 |
| NCBI Gene ID: | 22933 |
| NCBI Accession: | Q8IXJ6.2 |
| UniProt Secondary Accession: | Q8IXJ6,O95889, Q924Y7, Q9P0G8, Q9UNT0, Q9Y6E9, A8K3V1 B2RB45, U5TP13, |
| UniProt Related Accession: | Q8IXJ6 |
| Molecular Weight: | 35,654 Da |
| NCBI Full Name: | NAD-dependent protein deacetylase sirtuin-2 |
| NCBI Synonym Full Names: | sirtuin 2 |
| NCBI Official Symbol: | SIRT2 |
| NCBI Official Synonym Symbols: | SIR2; SIR2L; SIR2L2 |
| NCBI Protein Information: | NAD-dependent protein deacetylase sirtuin-2 |
| UniProt Protein Name: | NAD-dependent protein deacetylase sirtuin-2 |
| UniProt Synonym Protein Names: | Regulatory protein SIR2 homolog 2; SIR2-like protein 2 |
| Protein Family: | NAD-dependent protein deacetylase |
| UniProt Gene Name: | SIRT2 |
| UniProt Entry Name: | SIR2_HUMAN |