| UniProt Protein Function: | Catalyzes the transfer of a single N-acetylglucosamine from UDP-GlcNAc to a serine or threonine residue in cytoplasmic and nuclear proteins resulting in their modification with a beta-linked N-acetylglucosamine (O-GlcNAc). Glycosylates a large and diverse number of proteins including histone H2B, AKT1, EZH2, PFKL, KMT2E/MLL5, MAPT/TAU and HCFC1. Can regulate their cellular processes via cross-talk between glycosylation and phosphorylation or by affecting proteolytic processing. Involved in insulin resistance in muscle and adipocyte cells via glycosylating insulin signaling components and inhibiting the 'Thr-308' phosphorylation of AKT1, enhancing IRS1 phosphorylation and attenuating insulin signaling. Involved in glycolysis regulation by mediating glycosylation of 6-phosphofructokinase PFKL, inhibiting its activity (PubMed:22923583). Component of a THAP1/THAP3-HCFC1-OGT complex that is required for the regulation of the transcriptional activity of RRM1. Plays a key role in chromatin structure by mediating O-GlcNAcylation of 'Ser-112' of histone H2B: recruited to CpG-rich transcription start sites of active genes via its interaction with TET proteins (TET1, TET2 or TET3) (PubMed:22121020, PubMed:23353889). As part of the NSL complex indirectly involved in acetylation of nucleosomal histone H4 on several lysine residues (PubMed:20018852). O-GlcNAcylation of 'Ser-75' of EZH2 increases its stability, and facilitating the formation of H3K27me3 by the PRC2/EED-EZH2 complex (PubMed:24474760). Regulates circadian oscillation of the clock genes and glucose homeostasis in the liver. Stabilizes clock proteins ARNTL/BMAL1 and CLOCK through O-glycosylation, which prevents their ubiquitination and subsequent degradation. Promotes the CLOCK-ARNTL/BMAL1-mediated transcription of genes in the negative loop of the circadian clock such as PER1/2 and CRY1/2 (PubMed:12150998, PubMed:18288188, PubMed:19377461, PubMed:19451179, PubMed:20018868, PubMed:20200153, PubMed:21285374, PubMed:15361863). |
| NCBI Summary: | This gene encodes a glycosyltransferase that catalyzes the addition of a single N-acetylglucosamine in O-glycosidic linkage to serine or threonine residues. Since both phosphorylation and glycosylation compete for similar serine or threonine residues, the two processes may compete for sites, or they may alter the substrate specificity of nearby sites by steric or electrostatic effects. The protein contains multiple tetratricopeptide repeats that are required for optimal recognition of substrates. Alternatively spliced transcript variants encoding distinct isoforms have been found for this gene. [provided by RefSeq, Oct 2009] |
| UniProt Code: | O15294 |
| NCBI GenInfo Identifier: | 68067509 |
| NCBI Gene ID: | 8473 |
| NCBI Accession: | O15294.3 |
| UniProt Secondary Accession: | O15294,Q7Z3K0, Q8WWM8, Q96CC1, Q9UG57, |
| UniProt Related Accession: | O15294 |
| Molecular Weight: | 74,536 Da |
| NCBI Full Name: | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit |
| NCBI Synonym Full Names: | O-linked N-acetylglucosamine (GlcNAc) transferase |
| NCBI Official Symbol: | OGT |
| NCBI Official Synonym Symbols: | HRNT1; HINCUT-1; O-GLCNAC |
| NCBI Protein Information: | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit |
| UniProt Protein Name: | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit |
| UniProt Synonym Protein Names: | O-GlcNAc transferase subunit p110; O-linked N-acetylglucosamine transferase 110 kDa subunit; OGT |
| Protein Family: | Methylated-DNA--protein-cysteine methyltransferase |
| UniProt Gene Name: | OGT |
| UniProt Entry Name: | OGT1_HUMAN |

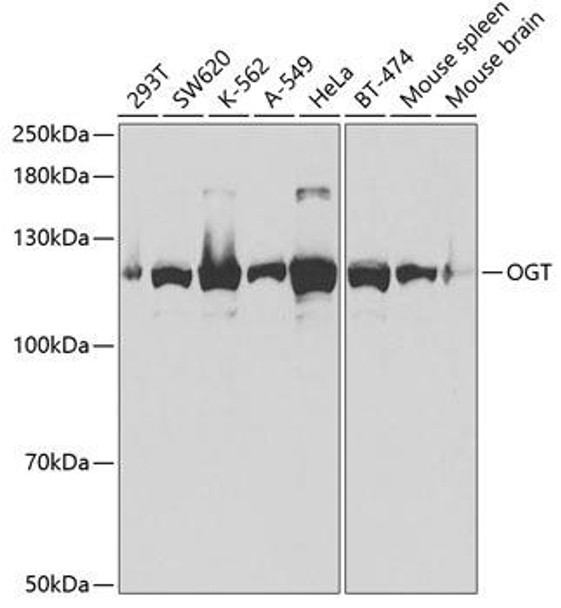
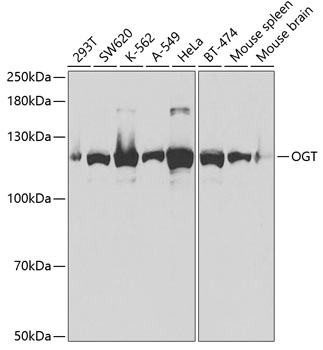
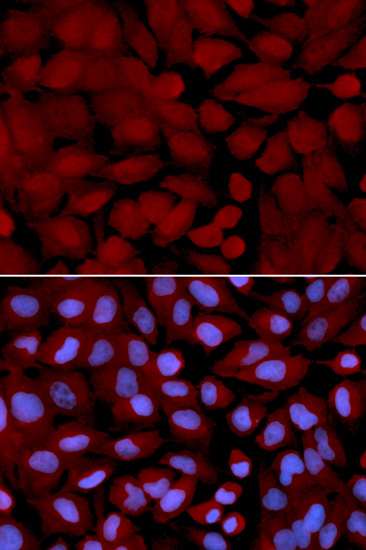
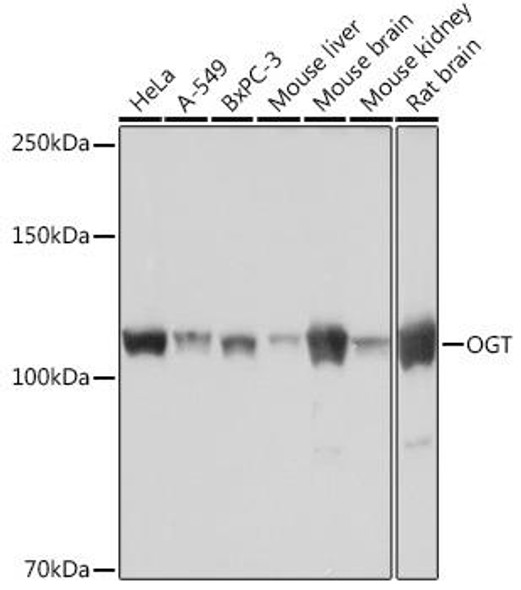
![Anti-M6PR Antibody (CAB19907)[KO Validated] Anti-M6PR Antibody (CAB19907)[KO Validated]](https://cdn11.bigcommerce.com/s-39x6lpnvxv/images/stencil/590x590/products/32642/30831/anti-m6pr-antibody-cab19907ko-validated__46230__03749.1644317723.jpg?c=1)
![Anti-FIBP Antibody (CAB19908)[KO Validated] Anti-FIBP Antibody (CAB19908)[KO Validated]](https://cdn11.bigcommerce.com/s-39x6lpnvxv/images/stencil/590x590/products/19236/17436/anti-fibp-antibody-cab19908ko-validated__86376__64836.1644251927.jpg?c=1)
![Anti-MDH2 Antibody (CAB19906)[KO Validated] Anti-MDH2 Antibody (CAB19906)[KO Validated]](https://cdn11.bigcommerce.com/s-39x6lpnvxv/images/stencil/590x590/products/21583/19783/anti-mdh2-antibody-cab19906ko-validated__22855__88853.1644252935.jpg?c=1)
![Anti-MPP2 Antibody (CAB19909)[KO Validated] Anti-MPP2 Antibody (CAB19909)[KO Validated]](https://cdn11.bigcommerce.com/s-39x6lpnvxv/images/stencil/590x590/products/21584/19784/anti-mpp2-antibody-cab19909ko-validated__25210__73359.1644252936.jpg?c=1)